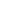Inspection History by Key Global Health Authorities
2023
US FDA inspection with no 483
2023
PMDA inspection
2023
NMPA inspection
2022
TGA inspection
2022
KFDA inspection
2022
NMPA inspection
2020
PMDA inspection
2020
NMPA inspection
2019
EMA inspection
2018
NMPA inspection
2017
US FDA inspection with no 483
2015
NMPA inspection
2010
NMPA inspection
2007
BfArM inspection
2005
NMPA inspection