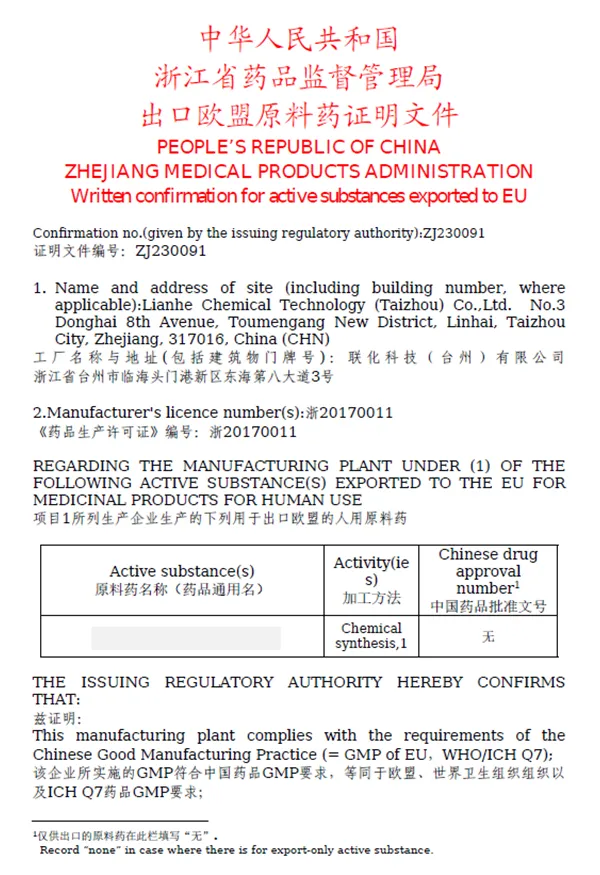
We understand that your project requires teamwork, know-how and experience. Our technology platform can fulfill your specific needs.
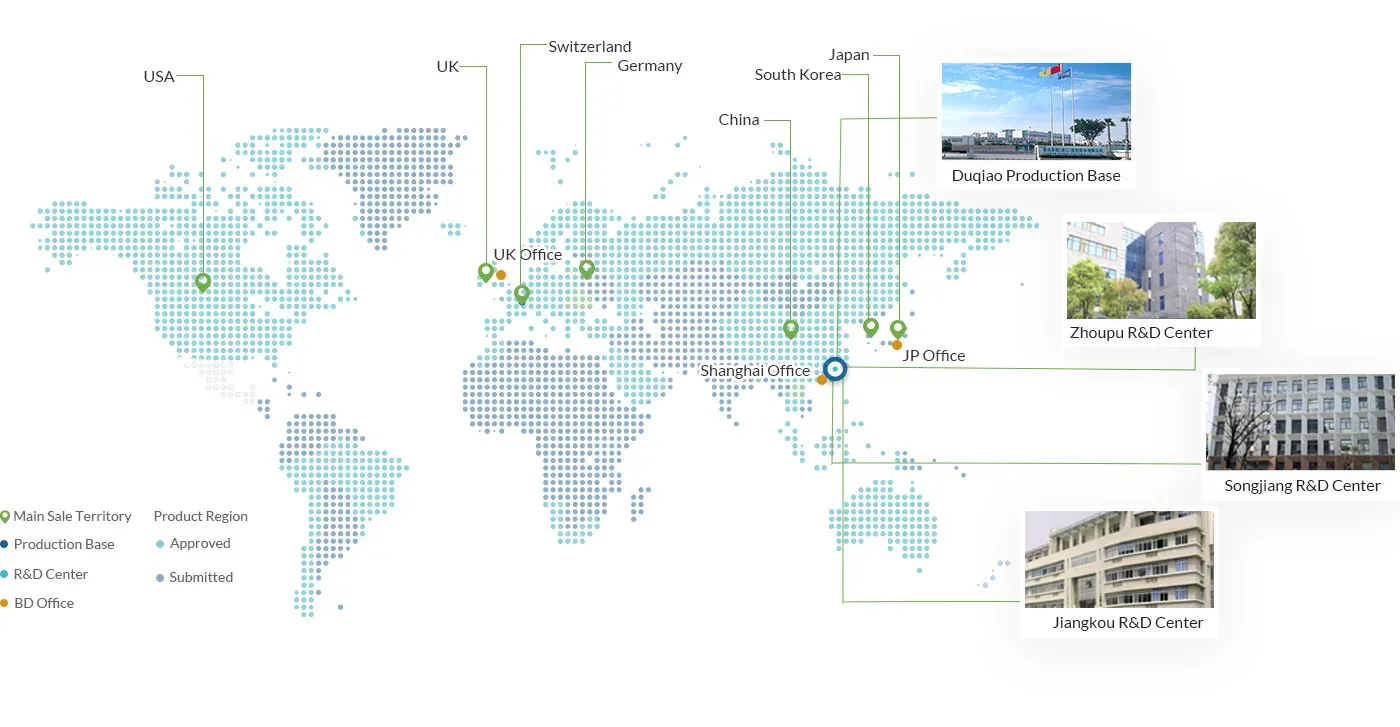
Lianhe Aigen Pharma Co., Ltd. is a leading provider of innovative drug solutions at home and abroad. Shanghai R&D center, Lianhe Aigen Pharmatech Co., Ltd., as a CRO platform, provides preclinical new drug discovery services for global customers. Taizhou Site, Lianhe Aigen Pharma Co., Ltd. (formerly known as Lianhe Chemical Technology (Taizhou) Co., Ltd.), as a CDMO platform.

1500+
Employees
200+
Scientist
25+
Years of Experience
60%
Top 20 Big Pharm
6
Key Global Health Authorities